Abstract
Background
There is increasing evidence that PI3K/AKT/mTOR signalling plays an important role in the tumour biology of diffuse large B cell lymphoma (DLBCL) (Wanner et al. Br. J. Haematol. 2006). Rapamycin analogues, such as everolimus and temsirolimus, have an overall response rate (ORR) of 28-30% in relapsed / refractory (r/r) DLBCL, although this is short lived, with a median progression free survival (PFS) of 2.6 months (Witzig et al. Leukaemia 2011; Smith et al. JCO 2010). Rituximab may have an additive effect with improvement in ORR to 38% (Barnes et al. Haematologica 2013). A number of resistance mechanisms to mTORC1 inhibitors have been elucidated, including phosphorylation of AKT by mTORC2; mTORC2 is not inhibited by rapamycin analogues. Pre-clinical data suggest that dual inhibition of mTORC1/2 may overcome this resistance (Gupta et al. Blood 2012). AZD2014 (vistusertib) is a potent and specific dual mTORC1/2 inhibitor with superior pharmacokinetics compared with previously developed dual mTOR inhibitors (Pike et al. Bioorg Med Chem Lett 2013). Using an intermittent dosing schedule, it was well-tolerated in a phase I trial in solid tumours (Banerji et al. JCO 2012).
Methods
The 'TORCH' trial was a prospective, single-arm, multicentre phase II trial using a two-stage design. Patients had to have r/r DLBCL after at least 1 course of potentially curative chemo-immunotherapy and be either ineligible for, or have relapsed post, autologous stem cell transplant (ASCT). For the first stage (n = 30), patients received vistusertib monotherapy 125mg BD on an intermittent schedule (2 days on treatment, 5 days off). For the second stage (n = 6), rituximab 375mg/m2 was additionally administered on day 1 of a 28-day cycle for 6 cycles. PET-CT scans were performed after cycle 2, 6 and at disease progression. Treatment was ongoing until progression, unacceptable toxicity or patient choice. The primary outcome was best ORR during the first 6 months. Secondary outcomes were tolerability of vistusertib monotherapy, and in combination with rituximab, overall survival (OS), PFS, duration of response (DOR) and change in the sum of the product of diameters (SPD). Exploratory outcomes included assessment of response by cell of origin and assessment of mTOR pathway by immunohistochemistry. The effect of dual mTORC1/2 inhibition on the sensitivity of PET was also assessed. Since the PI3K/AKT/mTOR pathway promotes glucose uptake in response to insulin, inhibitors of the pathway may disproportionately switch off PET signal in FDG-avid disease (Ma et al. JCO 2009).
Results
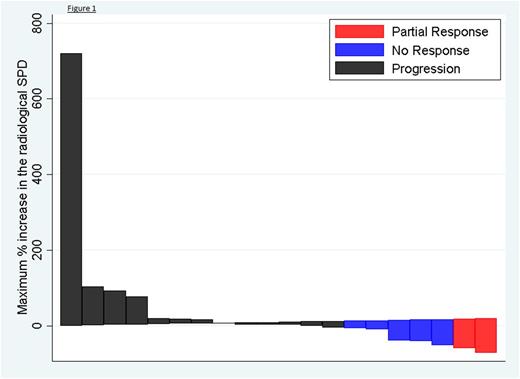
30 patients were recruited to the single-agent first stage, 6 patients to the rituximab-combination second stage across 9 centres. The median age of patients recruited was 68 years (range 33 to 82). 53% patients had high IPI scores (≥3). 68% of 31 patients evaluable by immunohistochemistry were of GCB subtype, 32% of non-GCB subtype. The median number of prior lines of chemotherapy was 3 (range 1 to 6). 47% patients were primary refractory. 17% patients had undergone prior ASCT. 5.6% of patients achieved a partial remission (PR), with no complete remissions (CR) and 19.4% of patients had stable disease (SD). 41.8% patients discontinued treatment prior to response assessment. Responding patients were of GCB and T-cell rich DLBCL subtypes. PET/CT and CT responses were concordant. Change in the SPD is demonstrated in the waterfall plot below (figure 1). Median PFS was 1.9 (95% CI 1.61, 2.42) months with 3 patients continuing on treatment at the time of data cut off. The median OS was 4.6 (95% CI 2.42, 7.13) months. Patients who responded had a median DOR of 57 days. The most common adverse events (AEs) were fatigue (56%), mucositis (33%), diarrhoea (50%) and nausea (47%). 87% of AEs were G1-2.
Conclusions
The 'TORCH' trial shows that single-agent, dual mTORC1/2 inhibitors do not appear to confer a significant advantage over mTORC1 inhibitors in r/r DLBCL, albeit in a high-risk patient cohort. This suggests that mechanisms other than mTORC2 escape, such as ERK/MEK/MNK activation, or enhanced upstream signalling through PI3K are important in mediating resistance. The use of an intermittent dosing schedule may have also negatively impacted on response. However, the 'TORCH' trial demonstrates that dual mTORC1/2 inhibitors are well-tolerated and can potentially be safely combined with rituximab. Other combinations are also being tested e.g. BTK inhibitor - NCT03205046.
Hildyard: ADC Therapeutics: Research Funding. Pettitt: Napp: Research Funding; GSK/Novartis: Research Funding; Gilead: Honoraria, Research Funding; Chugai: Research Funding; Celgene: Research Funding; AstraZeneca: Research Funding; Roche: Research Funding. Rule: TG Therapeutics: Consultancy, Honoraria; Napp: Consultancy; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Pharmacyclics: Consultancy, Honoraria; Kite: Consultancy; Sunesis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Astra-Zeneca: Consultancy, Honoraria. Cwynarski: Roche: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Conference expenses; Janssen: Other: Conference expenses. Barrington: Roche: Consultancy, Speakers Bureau; Hermes Medical Solutions: Research Funding; Siemens Medical: Research Funding; AstraZeneca: Research Funding. Wrench: Roche: Honoraria, Speakers Bureau. Davies: GSK: Research Funding; Janssen: Honoraria, Research Funding; CTI: Consultancy, Honoraria, Other: Travel expenses to attend conference; Pfizer: Honoraria, Research Funding; Celgene: Honoraria, Other: Advisory Board, travel expenses to attend conference, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel expenses to attend conference, Research Funding; Bayer: Research Funding; Acerta Phamra: Research Funding; Karyopharma: Honoraria, Research Funding; Seattle Genetics: Research Funding. Collins: Roche: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Amgen: Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; Celleron: Consultancy; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding; ADC Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal